Give the Full Electron Configuration for Sulfur.
The electron configuration of a neutral sulfur atom will thus be S. See the answer See the answer done loading.

Find The Electron Configuration For Sulfur S And The Sulfide Ion S2 Youtube
The next six electrons will go in the 2p orbital.

. Which electron configuration is an atom in an excited state. This problem has been solved. The Shorthand electron configuration or Noble gas configuration as well as Full electron configuration is also mentioned in the table.
1s2 2s2 2p6 3s2 3p4 Now the sulfide anion S2- is formed when two electrons are added to a neutral sulfur atom. Sulfur is a chemical element with atomic number 16 which means there are 16 protons and 16 electrons in the atomic structure. Sulfur is an electronegative element.
Who are the experts. Well put six in the 2p orbital and then put the next two. Since 1s can only hold two electrons the next 2 electrons for Calcium go in the 2s orbital.
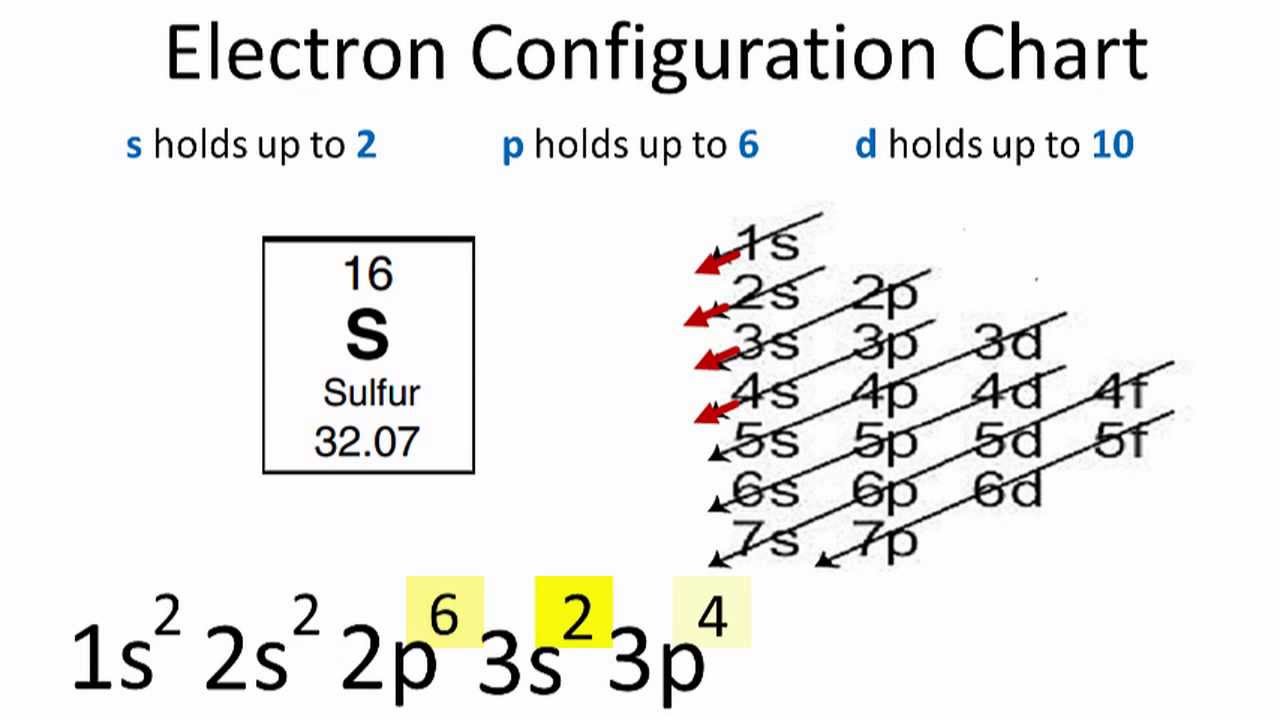
Sulfurs atomic number is 16. 1s2 2s2 2p6 3s2 3p1. In writing the electron configuration for Sulfur the first two electrons will go in the 1s orbital.
See the answer. What is the full electron configuration of sulfur. The number of unpaired electrons in the last orbit of an element is the valency of that element.
Give the full electron configuration for sulfur. Therefore it has 16 electrons in its outermost energy level. Therefore the Iron electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6.
The next six electrons will go in the 2p orbital. In writing the electron configuration for Chlorine the first two electrons will go in the 1s orbital. 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 6 7s 2 5f 14 6d 10 7p 6 Alternatively write the symbol for the noble gas before an element radon in this case and just add the extra information.
Since 1s can only hold two electrons the next 2 electrons for Chlorine go in the 2s orbital. We now shift to the 4s orbital where we place the remaining two electrons. Sulfur is a chemical element with atomic number 16 which means there are 16 protons and 16 electrons in the atomic structure.
The electron configuration of sulfur excited state is S 16 1s 2 2s 2 2p 6 3s 2 3p x1 3p y1 3p z1 3d xy1. Possible oxidation states are 46-2. 119 rows Electron Configuration Chart of All Elements Full Chart January 1 2022 March 7 2021 by Admin.
Give the full electron configuration for sulfur. Sulfurs has an atomic number equal to 16 which means that a neutral sulfur atom has a total of 16 electrons surrounding its nucleus. So the remaining six electrons enter the 4p orbital.
Sulfur needs another two electrons to have a stable arrangement. The p orbital can hold up to six electrons. Its electron configuration is.
Electron Configuration and Oxidation States of Sulfur. The electron configuration for sulfur is 1s2 2s2 2p6 3s2 3p4. Since 1s can only hold two electrons the next 2 electrons for sulfur go in the 2s orbital.
The chemical symbol for Sulfur is S. The 3d orbital is now full of electrons. The p orbital can hold up to six electrons.
Ne3s23p1 Aluminium has atomic number 13 so the full electron configuration will be. See answer 1 Best Answer. Electron configuration of Sulfur is Ne 3s2 3p4.
The next six electrons will go in the 2p orbital. For this the valency valence of sulfur is 4. The electron configuration of sulfur excited state is S 16 1s 2 2s 2 2p 6 3s 2 3p x1 3p y1 3p z1 3d xy1.
The p orbital can hold up to six electrons. Chemistry questions and answers. See the answer See the answer done loading.
1s2 2s2 2p6 3s2. This is the best answer based on feedback and ratings. Note that when writing the electron configuration for an atom like Fe the 3d is usually.
Well put six in the 2p orbital and then put the next two. Electron configuration chart of all Elements is mentioned in the table below. After the 4s is full we put the remaining six electrons in the 3d orbital and end with 3d6.
Give the full electron configuration for sulfur. So the next two electrons will enter the 4s orbital and ten electrons will enter the 3d orbital. In writing the electron configuration for Calcium the first two electrons will go in the 1s orbital.
1s2 2s2 2p6 3p1. Here sulfur has four unpaired electrons. Sulfur is the SIXTH element in the THIRD row of the periodic tableSo it is the same as Neon but with a full 3s2 subshell and four electrons in its 3pWhen.
Krypton Kr electron configuration. It is 1s2 2s2 2p6 3s2 3p4 Wiki. Solved Give the full electron configuration for sulfur.
1s2 2s2 2p6 3s2 3p2. And the 3s2 3p4 isnt the right answer. Therefore the krypton Kr electron configuration will be 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6.
SulfurS is the 16th element in the periodic table and its symbol is S. 100 39 ratings 1s2. The sulfurS electron configuration will be 1s2 2s2 2p6 3s2 3p4.

Sulfur Electron Configuration Youtube

No comments for "Give the Full Electron Configuration for Sulfur."
Post a Comment